Atlantic Canada Poison Centre
Antidote Kit Manual
Acetylcysteine - Traditional
Parvolex, Mucomyst, N-Acetylcysteine, NAC
ALERT: NS / PEI / NB hospitals: Please use "Acetylcysteine - Single Concentration". Do NOT use traditional protocol.
Indications
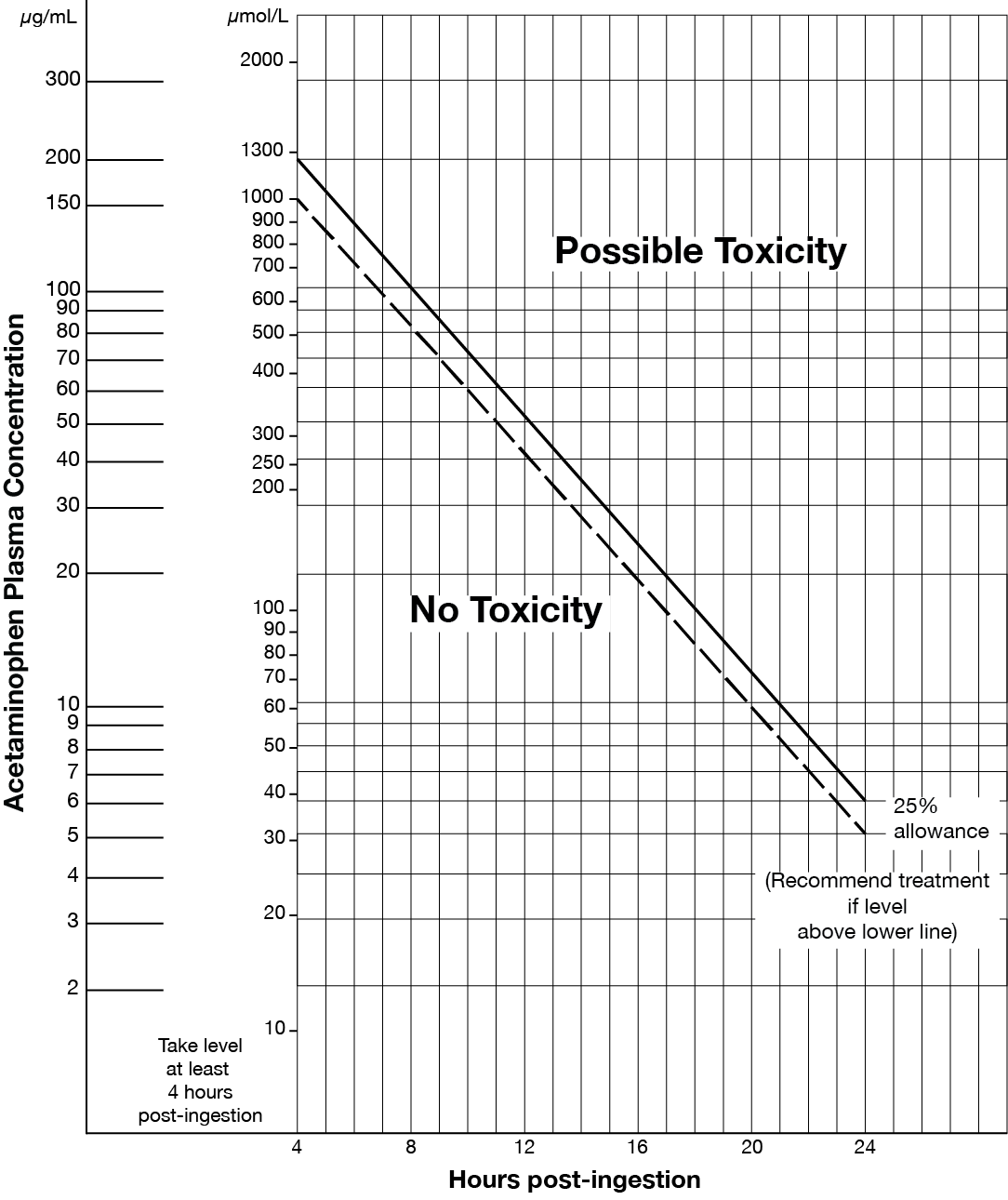
- Acute acetaminophen overdose (refer to Matthew-Rumack nomogram at end of monograph).
- Repeated supratherapeutic (chronic) acetaminophen ingestion.
- Fulminant hepatic failure due to acetaminophen overdose or other hepatotoxic toxins.
Dosage
A) Intravenous 21-hour protocol
|
Acetylcysteine |
Dilution |
Duration of Infusion |
|
First Infusion Up to maximum dose of 15 grams |
Add dose of acetylcysteine to 2.25 mL/kg of IV fluid. Final concentration of 50 mg/mL |
1 hour |
|
Second Infusion Up to maximum dose of 5 grams |
Add dose of acetylcysteine to 4.75 mL/kg of IV fluid final concentration of 10 mg/mL |
4 hours |
|
Third Infusion Up to maximum dose of 10 grams |
Add dose of acetylcysteine to 9.5 mL/kg of IV fluid. Final concentration of 10 mg/mL |
16 hours † |
B) ORAL 20 HOUR PROTOCOL
Rarely, oral dosing is necessary for patients who develop refractory anaphylactoid reactions to intravenous acetylcysteine. (Refer to Potential Hazards section below)
Loading dose: 140 mg/kg (0.7 mL/kg of 20% acetylcysteine solution). Dilute with soda/juice (2 mL/kg).
Maintenance doses: 70 mg/kg (0.35 mL/kg of 20% acetylcysteine solution) given every 4 hours for five doses. Dilute with soda/juice (1 mL/kg).
|
Criteria for Discontinuing NAC (IV or Oral)
At the end of the 21 h protocol, NAC may be discontinued if all of the following criteria are met: negative acetaminophen level, INR less than or equal to 1.5, AST or ALT less than 50 IU/L OR, if elevated, declining and approximately 50% of the peak value measured. If any of these criteria are not met, continue the NAC at the same rate as the continuous infusion. Bloodwork should then be done every 4 to 12 hours, depending on the clinical scenario. As soon as criteria for stopping NAC is reached, NAC can be discontinued.
Administration
- Intravenous 21-hour protocol: Dilute dose in dextrose 5% in water (most common) or sodium chloride 0.9% and administer according to protocol provided in the “dosage” section.
- Oral: Calculate and dilute dose according to protocol provided in the “dosage” section.
Compatibility, Stability
- Stable for 24 hours diluted in dextrose 5% in water or sodium chloride 0.9% at room temperature.
Potential Hazards of Administration
- Nausea, vomiting: can be treated with regular anti-nauseant medications if necessary.
- Monitor heart rate and blood pressure before, during and after the infusion.
- Anaphylactoid reactions (intravenous dosing). Patients should be monitored closely during the first hour of drug administration. Symptoms include: pruritus, rash, facial edema, urticaria, flushing, chest tightness, tachycardia, hypotension and bronchospasm. Anaphylactoid reactions may be related to the rate of administration and often occur during the loading dose. If anaphylactoid symptoms appear, temporarily discontinue the infusion and assess the patient. Administer an antihistamine if required. If the reaction is severe, follow usual practice for treatment of an allergic reaction. Restart the infusion at a slower rate once the patient is stabilized (i.e., reduce the rate of infusion by half). It is not usually necessary to discontinue acetylcysteine. The entire amount of the acetylcysteine must be administered.
Miscellaneous
- For acute acetaminophen overdose, acetylcysteine is of maximal benefit if initiated within 8 hours post ingestion but is beneficial at greater than 24 hours after ingestion.